| 氯化亚铁四水合物 基本信息 | |||
|---|---|---|---|
| CAS号 | 13478-10-9 | 分子式 | Cl2FeH8O4 |
| 分子量 | 198.81200 | 精确质量 | 197.91500 |
| PSA | 36.92000 | LogP | 1.11930 |
 基本信息
展开↓
基本信息
展开↓  生产制备方法及用途展开↓
生产制备方法及用途展开↓
1.先用相对密度为1.18的盐酸配制成27%左右的盐酸溶液,然后缓慢加入铁屑,进行反应。
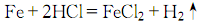
当氢气不再生成时,冷却,过滤,滤液中加入一些便面洗净的小铁片,以防止氯化亚铁的氯化,然后加热蒸发浓缩至结晶薄膜,趁热过滤,滤液冷却结晶,以下迅速干燥,制的氯化亚铁结晶。无水氯化亚铁可用铁与气态氯化氢在900 ºC下反应制的。
2.在12.3℃以上的温度及无氧条件下,将铁溶于盐酸时可制得氯化亚铁的四水合物。
1.用作分析试剂、还原剂及媒染剂。
2.用于冶金工业。用作媒染剂、分析试剂,并用于医药、冶金和污水处理等。
 物化性质展开↓
物化性质展开↓
1.常温常压下稳定
2.避免的物料 氧化物。易潮解。在空气中易被氧化。溶于水和乙醇。微溶于丙酮,溶于乙醇,易溶于水。
1. 性状:蓝绿色单斜晶体,易潮解。
2. 密度(g/mL,25/4℃):1.93
3. 相对蒸汽密度(g/mL,空气=1):未确定
4. 熔点(ºC):670-674
5. 沸点(ºC,常压):未确定
6. 沸点(ºC,5.2kPa):未确定
7. 折射率:未确定
8. 闪点(ºC):未确定
9. 比旋光度(º):未确定
10. 自燃点或引燃温度(ºC):未确定
11. 蒸气压(kPa,25ºC):未确定
12. 饱和蒸气压(kPa,60ºC):未确定
13. 燃烧热(KJ/mol):未确定
14. 临界温度(ºC):未确定
15. 临界压力(KPa):未确定
16. 油水(辛醇/水)分配系数的对数值:未确定
17. 爆炸上限(%,V/V):未确定
18. 爆炸下限(%,V/V):未确定
19. 溶解性:可溶解与水和乙醇。
 安全信息展开↓
安全信息展开↓  毒理性展开↓
毒理性展开↓
1、急性毒性:小鼠口经LC50:450 mg/kg;
主要的刺激性影响:在皮肤上面:刺激皮肤和粘膜;在眼睛上面:强烈的刺激性和造成严重伤害眼睛的危险,刺激的影响;
2、致敏作用:通过皮肤接触可能造成敏化作用。
通常对水体是稍微有害的,不要将未稀释或大量产品接触地下水,水道或污水系统,未经政府许可勿将材料排入周围环境。
 MSDS 展开↓
MSDS 展开↓| Name: | Iron(II) chloride tetrahydrate 99+% Material Safety Data Sheet |
| Synonym: | Ferrous chloride tetrahydrat |
| CAS: | 13478-10-9 |
| CAS# | Chemical Name | content | EINECS# |
| 13478-10-9 | Iron (II) chloride | 99+% | unlisted |
 分子结构与计算化学数据展开↓
分子结构与计算化学数据展开↓
1、摩尔折射率:无可用的
2、摩尔体积(cm3/mol):无可用的
3、等张比容(90.2K):无可用的
4、表面张力(dyne/cm):无可用的
5、介电常数:无可用的
6、极化率(10-24cm3):无可用的
7、单一同位素质量:197.914906 Da
8、标称质量:198 Da
9、平均质量:198.8121 Da
1、 氢键供体数量:4
2、 氢键受体数量:6
3、 可旋转化学键数量:0
4、 拓扑分子极性表面积(TPSA):4
5、 重原子数量:7
6、 表面电荷:0
7、 复杂度:3.6
8、 同位素原子数量:0
9、 确定原子立构中心数量:0
10、 不确定原子立构中心数量:0
11、 确定化学键立构中心数量:0
12、 不确定化学键立构中心数量:0
13、 共价键单元数量:7